You will need a copy of the periodic table! |
||||||||||||||||||||||||||||||||
1. When an atom forms a positive ion it ..
|
||||||||||||||||||||||||||||||||
16. The diagram shows the outline of part of the Periodic Table: Which two elements could form a covalent compound? |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
3. Four elements, K, L, M and N, have the following electronic configurations:
Which two elements react together to form a covalent compound?
|
||||||||||||||||||||||||||||||||
Q4-5:
|
|
|||||||||||||||||||||||||||||||
4. Which of the following statements best describes the formation of sodium fluoride?
|
||||||||||||||||||||||||||||||||
| 5. Which statement about the melting point of sodium fluoride is true?
Sodium fluoride has a ...
|
||||||||||||||||||||||||||||||||
| 6. Which statement best completes the description of covalent bonding?
Covalent bonding is the electrostatic attraction between ……….
|
||||||||||||||||||||||||||||||||
7. Lithium reacts with oxygen to form lithium oxide. The arrangement of the electrons in atoms of lithium and oxygen are shown in the diagram here. Which of the following dot and cross diagram shows the arrangement of the electrons in lithium oxide? |
|
|||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
8. Which of the following molecules contains 4 shared pairs of electrons?
|
||||||||||||||||||||||||||||||||
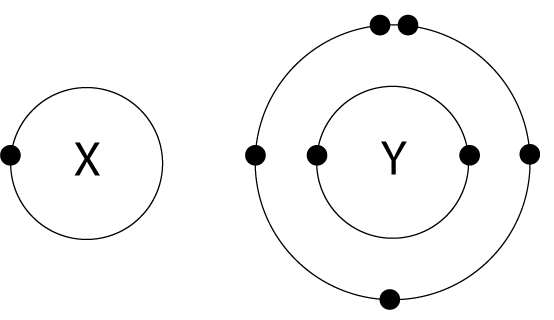
9. The arrangement of electrons in atoms X and Y are shown in the diagram here. X and Y form a covalent compound. What is the formula of the compound formed? |
 |
|||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
10. Two elements react to form an ionic compound with the formula MgCl2. The electronic configurations of the two elements before and after reaction are ..
|
||||||||||||||||||||||||||||||||