Properties & uses of metals, alloys and extraction of metals |
| 1. Which one of the following diagrams represents the structure of aluminium metal? |
|
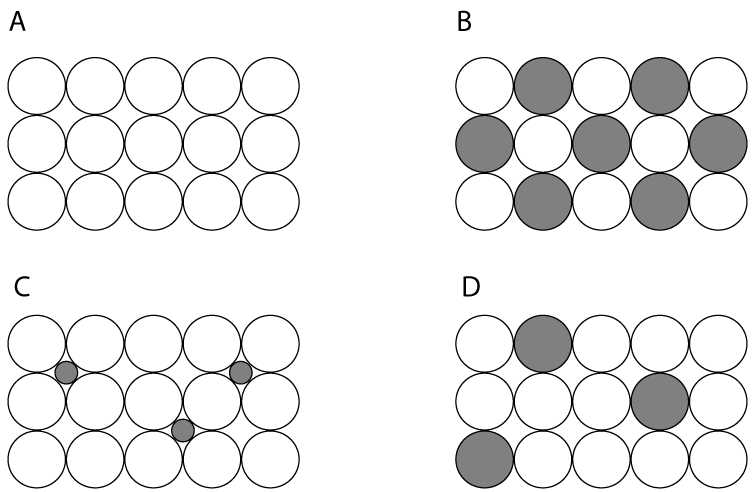 |
|
2. Aluminium is a very malleable metal and is used to make cooking foil.
Which statement explains why aluminium is malleable? |

Marco Verch | CC 2.0
|
- A. Aluminium atoms are arranged in layers so can slide over each other
- B. Aluminium atoms vibrate around fixed positions in the lattice
- C. Aluminium has strong metallic bonds which require a lot of energy to break
- D. Aluminium contains delocalized electrons which are free to move
|
|
3. Aluminium is used for food containers because it ...
- A. is a good conductor of electricity
- B. has a low density
- C. resists corrosion
- D. has a low melting point
|
|
4. Which one of the following statements best describes the structure of a metal?
- A. A giant lattice of atoms in a ‘sea of electrons’
- B. A giant lattice of atoms sharing electrons
- C. A giant lattice of positive and negative ions
- D. A giant lattice of positive ions in a ‘sea of electrons’
|
|
| 5. Aluminium alloys are used for airplanes because they ... |
 |
- A. are good conductors of electricity
- B. have a low density
- C. are good conductors of heat
- D. have a low melting point
|
|
| 6. Copper is the metal used in most electrical wiring because it is a very good conductor of electricity. |

Łukasz Klepaczewski | Pixabay |
Copper is a good conductor of electricity because it ..
- A. contains ions which are free to move
- B. contains atoms which are not free to move
- C. contains delocalized electrons which are free to move
- D. contains delocalized electrons which are not free to move
|
|
7. Alloys are typically stronger than pure metals because ...
- A. the metallic bonding is stronger in alloys
- B. the layers of metal atoms in the alloy cannot easily slide over each other
- C. alloys are covalently bonded and covalent bonds are strong
- D. the metals atoms are all very regularly arranged in alloys
|
|
8. Which of the following statements about steel is NOT correct?
- A. Stainless steel is used for cutlery as it does not rust
- B. Stainless steel is made by adding other elements to iron
- C. Mild steel is used for car bodies as it is strong and malleable
- D. Mild steel is a compound of iron and carbon
|
|
9. Mild steel is an alloy of iron and carbon. Which statement is true?
- A. The carbon makes the alloy softer than iron
- B. The carbon makes the alloy harder than iron
- C. The carbon stops the iron from rusting
- D. The carbon makes the alloy a better conductor than iron
|
|
10. Which property do ALL metals have?
- A. react with water
- B. low density
- C. conduct electricity
- D. react with dilute hydrochloric acid
|
|
|


