|
Target: 10 Questions in 10 minutes
An IB Chemistry data booklet is helpful |
||||||||||
1. For the reaction 2C2H2 (g) + 5O2 (g) → 4CO2 (g) + 2H2O(l), which of the following statements is correct?
| ||||||||||
2. Which one of the following statements is true about endothermic reactions?
|
||||||||||
3. A 27g aluminum block is heated from 18 oC to 68 oC and it absorbs 1215 J of energy. What is the molar specific heat capacity of aluminum in J mol-1 oC-1?
|
||||||||||
4. Which of the following reactions are exothermic?
|
||||||||||
| ||||||||||
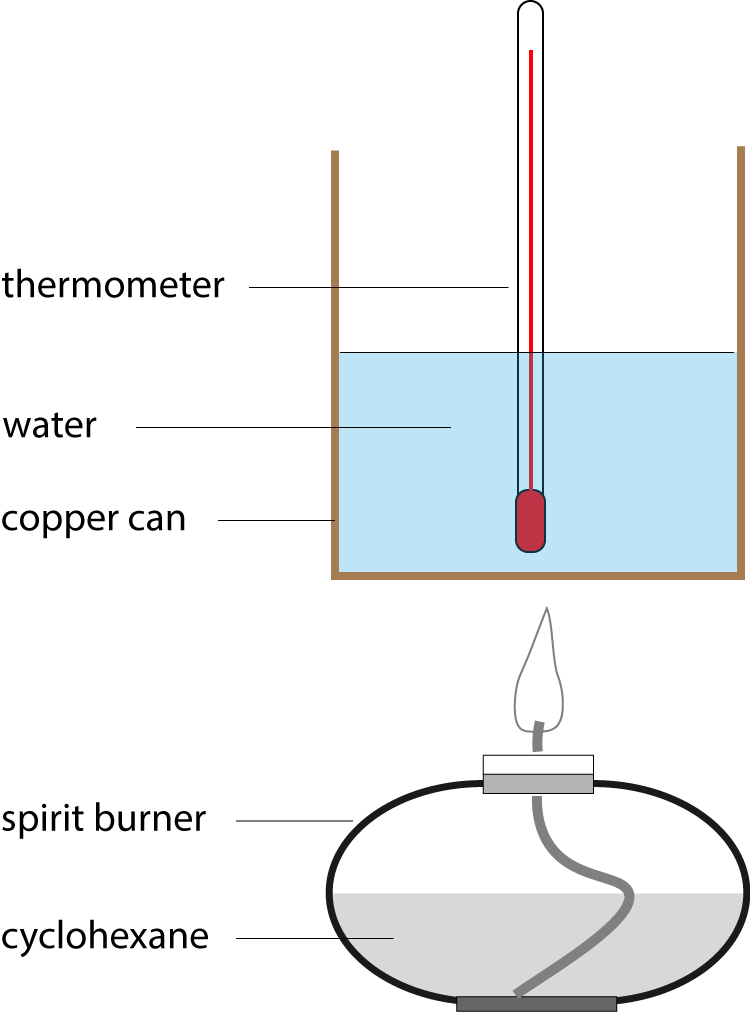
5. An experiment is carried out, as shown in the diagram, to measure the enthalpy change of combustion of cyclohexane. Burning 0.5 g of the fuel increases the temperature of 100g of water by 35 oC. The specific heat capacity of water is 4.18 J g-1 K-1. Which of the following expressions calculates the enthalpy change of combustion of cyclohexane in kJ mol-1? |
 |
|||||||||
| ||||||||||
6. The enthalpy change of combustion of butan-2-ol was determined experimentally using a calorimeter. The initial temperature of the water in the calorimeter was 23.0 °C, and after combusting for 90 seconds, it reached a maximum temperature of 60.0°C. The mass of the water in the calorimeter was 100.00 grams and the change in the mass of the alcohol was 0.700 g. If the specific heat capacity of water is 4.18 J g-1 °C-1, what is the measured enthalpy change of combustion of the alcohol in kJ mol-1?
|
||||||||||
7. The enthalpy change of solution of ammonium nitrate is given as +25.69 kJ mol-1. When 8.0 g of the salt is dissolved in 100 cm3 of water, what is the expected temperature change of the solution in oC? Assume the specific heat capacity of the solution is 4.2 J g-1 oC-1.
|
||||||||||
8. Calculate the amount of heat energy released when 100.0 cm3 of 0.400 mol dm-3 hydrochloric acid reacts with 25.0 cm3 of 0.800 mol dm-3 sodium hydroxide solution. The enthalpy change of the reaction HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) is -57 kJ mol-1 .
| ||||||||||
| 9. 3.00 g of zinc powder is measured with a scale and is added into 25 cm3 of 1 mol dm-3 copper(II) sulphate solution in a glass beaker. The starting temperature and maximum temperature of the reaction are measured using a digital thermometer, in order the calculate the enthalpy change of the reaction. | ||||||||||
What is the best way to reduce the random error in this experiment?
| ||||||||||
| 10. In an experiment to measure the enthalpy change of neutralization between potassium hydroxide and sulphuric acid, 50 cm3 of 2.00 mol dm-3 KOH(aq) was mixed with 25 cm3 of 1.50 mol dm-3 H2SO4(aq) in a polystyrene cup. The initial temperature of the solutions was 23.0 oC and after stirring, the maximum temperature reached was 36.3 oC. | ||||||||||
What is the enthalpy change of neutralization for this reaction in kJ mol-1, assuming that the density and specific heat capacity of all solutions are the same as for water?
|
||||||||||