|
Target: 10 Questions in 10 minutes
An IB Chemistry data booklet is helpful |
||||||||||
1. Which of the following statements about dynamic equilibrium is not correct?
| ||||||||||
| 2. Consider the reaction between H2 and I2 at equilibrium:
H2(g) + I2(g) ⇌ 2HI(g) ∆H = -13 kJ mol-1 Which of the following changes will shift the position of equilibrium to the left when applied to the system? |
||||||||||
|
||||||||||
| 3. In a reversible reaction between A(g) and B(g) to form C(g) and D(g)
aA(g) + bB(g) ⇌ cC(g) + dD(g) what is the correct expression for its equilibrium constant Kc? |
||||||||||
| ||||||||||
| 4. For the reversible reaction below, what is the effect of adding a catalyst on the position of equilibrium and the value of the equilibrium constant Kc?
CO2(g) + H2(g) ⇌ CO(g) + H2O(g) ∆H is positive |
||||||||||
| ||||||||||
5. Which of the following reactions will have its position of equilibrium shifted to the right when the pressure of the system increases?
|
||||||||||
| ||||||||||
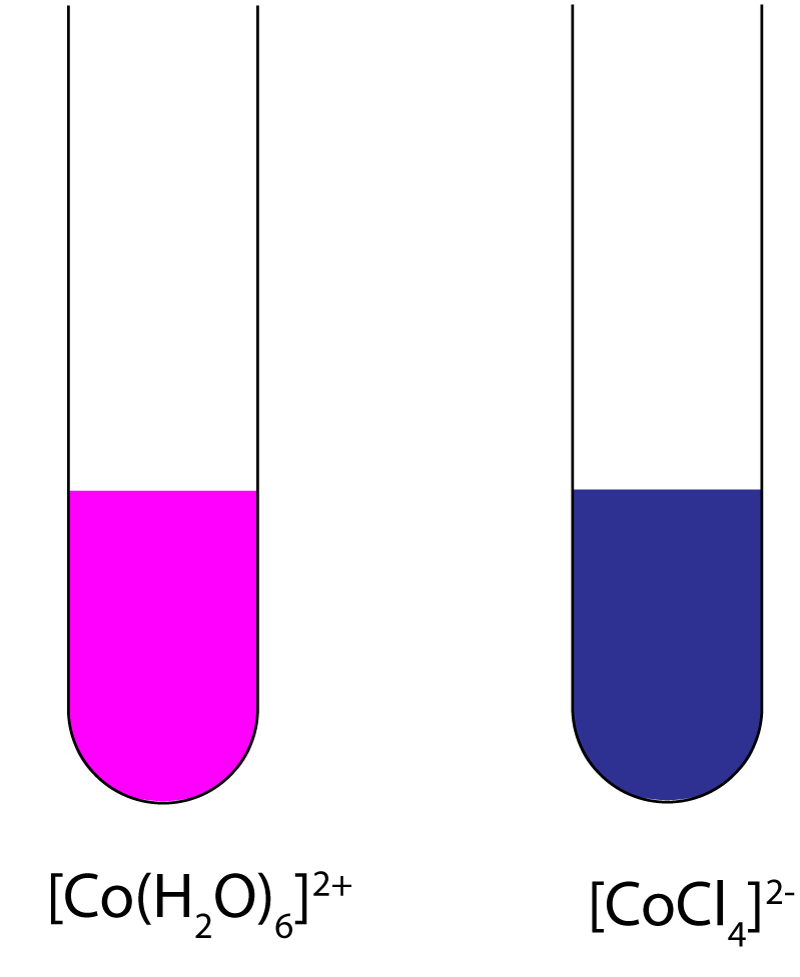
| 6. Consider the reaction:
[Co(H2O)6]2+(aq) + 4Cl-(aq) ⇌ [CoCl4]2-(aq) + 6H2O(l) |
||||||||||
|
The complex ion [Co(H2O)6]2+(aq) is pink in colour and the complex ion [CoCl4]2-(aq) is dark blue. The enthalpy change of the forward reaction is endothermic. When the reaction is at equilibrium and is subjected to the following changes, which one results in a shift of colour to darker blue? |
 |
|||||||||
| ||||||||||
2SO2(g) + O2(g) ⇌ 2SO3(g) has an equilibrium constant Kc at temperature T. What is the equilibrium constant for the reverse reaction 2SO3(g) ⇌ 2SO2(g) + O2(g), at the same temperature? |
||||||||||
| ||||||||||
8. When the reaction N2(g) + O2(g) ⇌ 2NO(g) is at equilibrium at 298 K, it has an equilibrium constant K = 4.5 x 10-31. NO(g) ⇌ ½N2(g) + ½O2(g), at the same temperature? |
||||||||||
| ||||||||||
| 9. For the following reaction:
NH3(g) ⇌ ${1}\over{2}$N2(g) + ${3}\over{2}$H2(g) ∆H = +46 kJ mol-1 What is the effect of decreasing temperature on the position of equilibrium and the value of the equilibrium constant Kc? |
||||||||||
| ||||||||||
10. For a system at equilibrium, which of the following statements is correct?
|
||||||||||