Chemistry µIB:Hybridization (AHL)
Structure 2.2: Hybridization, sigma and pi bonding
|
Target: 10 Questions in 10 minutes
An IB Chemistry data booklet is helpful |
||||||||||||||||||||||
1. Which of the following species does NOT contain a π bond?
|
||||||||||||||||||||||
2. Which type of hybridization for carbon atoms is present in a molecule of benzoic acid, C6H5COOH?
| ||||||||||||||||||||||
|
||||||||||||||||||||||
3. How many σ and π bonds are present in a molecule of propanone CH3COCH3?
|
||||||||||||||||||||||
| 4. Which statement is NOT true about the bonding in this molecule? | 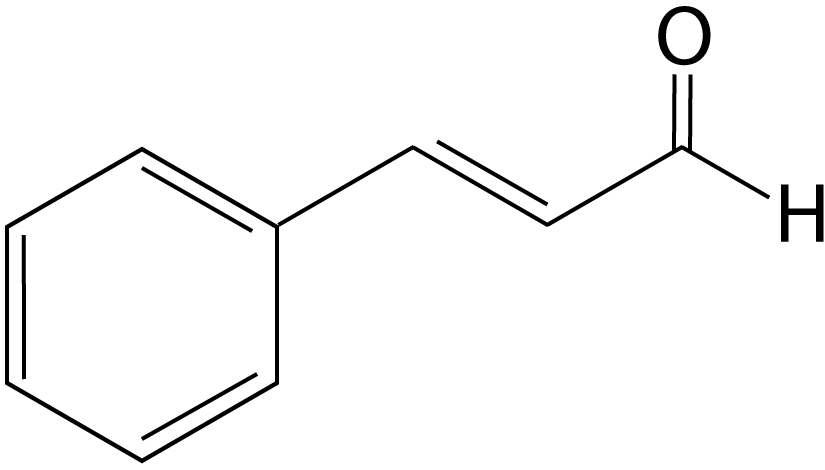 |
|||||||||||||||||||||
| ||||||||||||||||||||||
5. Which of the following molecules shows sp hybridization of an atom?
| ||||||||||||||||||||||
Q6-8: These questions are about the amino acid histidine shown here. Three atoms have been highlighted with circles: |
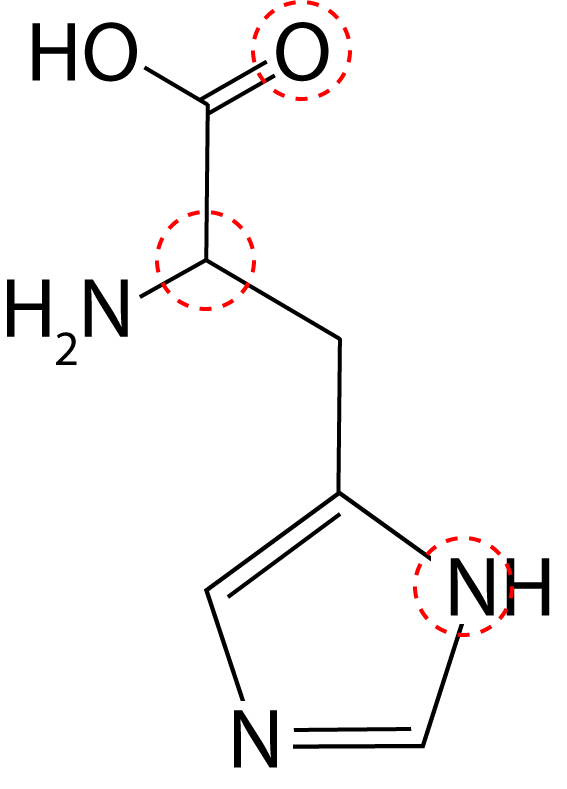 |
|||||||||||||||||||||
6. What are the hybridizations of the circled carbon, oxygen and nitrogen atoms?
| ||||||||||||||||||||||
7. How many σ bonds are there in a molecule of histidine?
| ||||||||||||||||||||||
8. How many π bonds are there in a molecule of histidine?
| ||||||||||||||||||||||
9.Which of the following molecules does NOT contain a sp2 hybridized nitrogen atom?
| ||||||||||||||||||||||
| 10. Which statement is true about the bonding in this molecule ? | 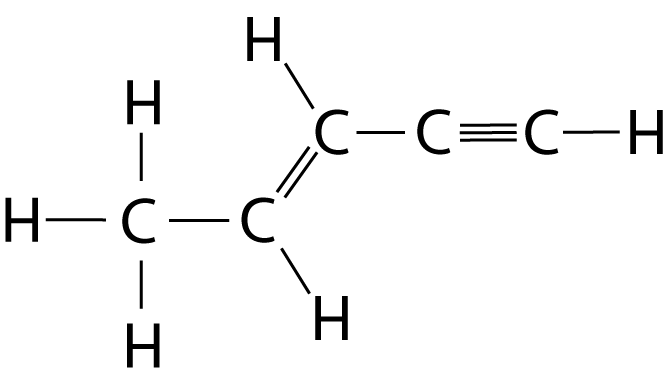 |
|||||||||||||||||||||
|
||||||||||||||||||||||