Chemistry µIB:Polar Molecules
Structure 2.2 (SL and HL)
|
Target: 10 Questions in 10 minutes
An IB Chemistry data booklet is helpful |
||||||||||||||||
Q1+2: The electronegativity values of 4 non-metal elements are listed below:
|
||||||||||||||||
1. Which of the covalent bonds below is most polar?
|
||||||||||||||||
2. Which bond polarity is correctly shown?
|
||||||||||||||||
3. Which bond is least polar?
| ||||||||||||||||
4. Which statement about covalent bonds is NOT true?
| ||||||||||||||||
Q5+6: These questions concern sulfur fluoride, SF2. 5. Which is the correct Lewis (electron dot) formula for SF2? |
||||||||||||||||
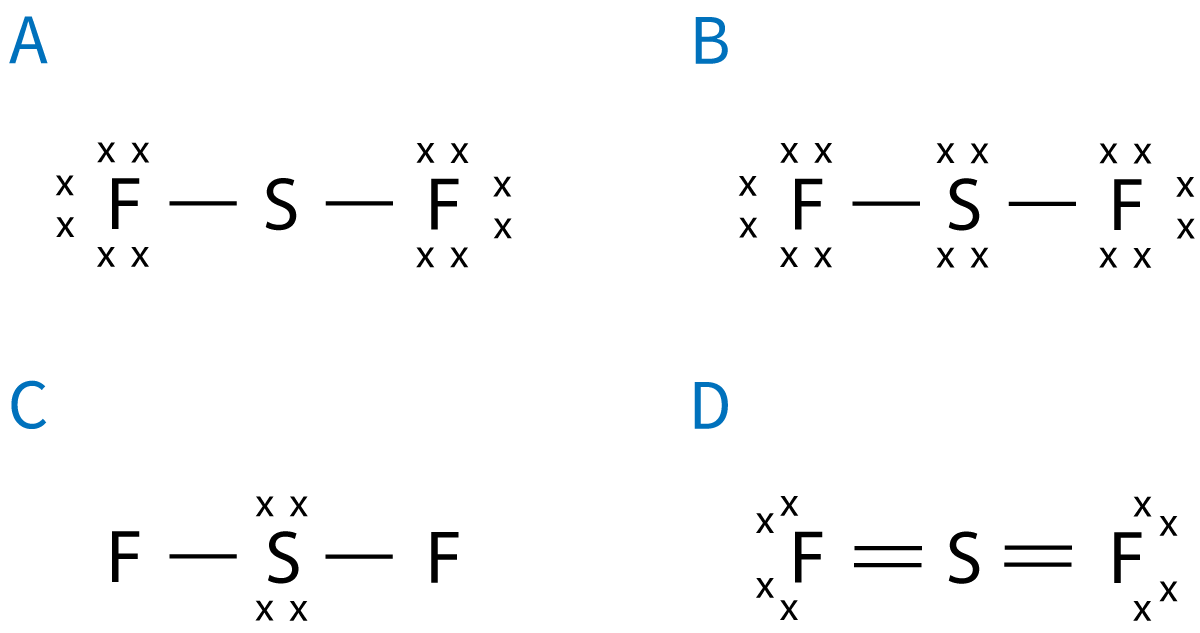 | ||||||||||||||||
6. What is the correct molecular shape for SF2 and will the molecule be polar or non-polar?
| ||||||||||||||||
7. Which statement is NOT true?
|
||||||||||||||||
8. Which molecule is polar?
|
||||||||||||||||
9. Which molecule has no net dipole moment?
| ||||||||||||||||
10.Which of the following substances contains polar covalent molecules?
|
||||||||||||||||