You will need a copy of the periodic table! |
1. The elements in the Periodic Table are arranged in order of..
- A. Relative Atomic Mass
- B. Mass Number
- C. Reactivity
- D. Atomic number
|
|
2. The group of elements which form ions with a 1+ charge is..
- A. group 1
- B. group 2
- C. group 6
- D. group 7
|
|
3. Which statement about elements in the same period of the Periodic Table is correct?
- A. they all react in a similar way
- B. they all have the same number of outer shell electrons
- C. they all have the same number of occupied shells
- D. they become more metallic as you move from left to right
|
|
4. Noble gases are unreactive because they..
- A. all have a full outer shell
- B. all have 8 electrons in their outer shell
- C. are all monatomic gases
- D. are in Group 0 of the Periodic Table
|
|
5. The element in group 2 and period 4 of the Periodic Table is..
- A. a metal with 4 outer shell electrons
- B. a metal with 2 outer shell electrons
- C. a non-metal with 4 outer shell electrons
- D. a non-metal with 2 outer shell electrons
|
|
6. The magnesium ion, Mg2+, has the same electronic configuration as an atom of..
- A. helium
- B. neon
- C. argon
- D. krypton
|
|
7. What property of elements increases as you move from left to right across the Periodic Table?
- A. number of electron shells
- B. metallic character
- C. number of outer shell electrons
- D. tendency to form positive ions
|
|
Q8-9:
A diagram of the Periodic Table is shown below. A, B, C and D are different elements.
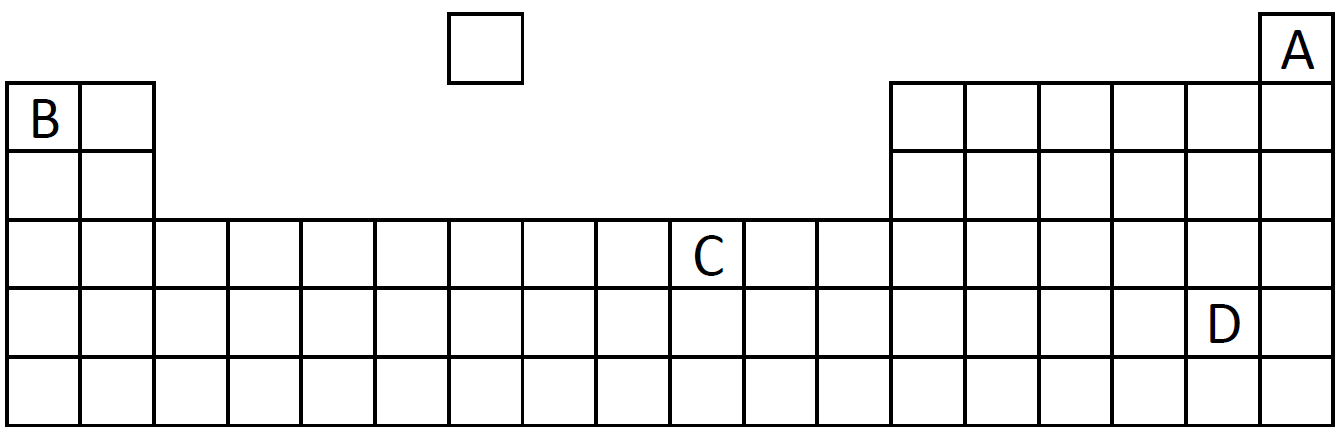
|
|
| 8. Which element exists as diatomic molecule? |
|
| 9. Which element has 2 electrons in its outer shell? |
|
10. Magnesium burns in air to form magnesium oxide. Magnesium oxide is..
- A. a basic oxide formed from a metal
- B. a basic oxide formed from a non-metal
- C. an acidic oxide formed from a metal
- D. an acidic oxide formed from a non-metal
|
|
|