|
10 minutes maximum! Can you do it in 5? |
||||||||||||||
1. Chemical reactions in which heat energy is given out are described as ...
| ||||||||||||||
2. The table below gives information about the temperature changes in 3 reactions:
|
||||||||||||||
The reactions which are exothermic are:
| ||||||||||||||
3. Which of the following types of chemical reactions are always endothermic?
| ||||||||||||||
Q4-6 refer to the following reaction profile:
|
||||||||||||||
4. This diagram represents the reaction profile for an ...
| ||||||||||||||
| 5. The enthalpy change (energy change) for this reaction is correctly labelled by line . | ||||||||||||||
6. Line D represents ...
|
||||||||||||||
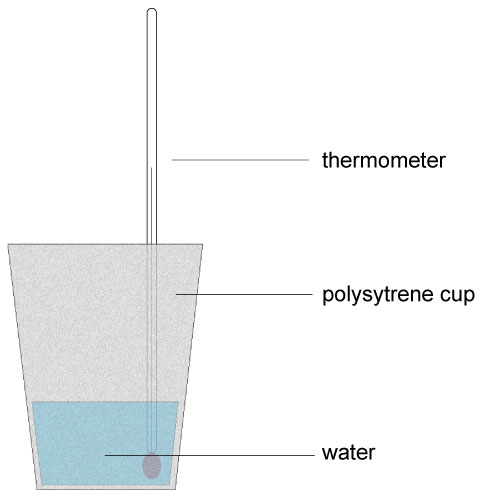
Q7 – 10. Water at 25 °C was used to dissolve ammonium nitrate (NH4NO3). The ammonium nitrate disappeared and a temperature of 21°C was recorded immediately afterwards. The student used a polystyrene cup to carry out this experiment as shown here. |
 |
|||||||||||||
7. The reason for using a polystyrene cup rather than a beaker is that a polystyrene cup is ...
| ||||||||||||||
8. The student correctly concluded that the dissolving of ammonium nitrate was ...
| ||||||||||||||
9. Which of the following energy level diagrams correctly represents this reaction?
| ||||||||||||||
10. Estimate the final temperature of the solution if the volume of water was halved but the same mass of ammonium nitrate used.
| ||||||||||||||