10 minutes maximum! Can you do it in 5? |
|||||||||||||||||
1.Which of the following answers best describes the strong nuclear force in the atom? |
|||||||||||||||||
|
|||||||||||||||||
2. Which of these statements gives the best definition of the 'binding energy' of a nucleus?
|
|||||||||||||||||
3. Which of the following 4 metals has the highest binding energy per nucleon?
|
|||||||||||||||||
4. The constant 'u' has a value of 931.5 MeV c-2. This unit MeV c-2 is a unit of...
| |||||||||||||||||
5. To calculate the energy released in fission and fusion, we use the formula: ΔE = Δm c2 What does the letter 'c' represent in this formula? |
|||||||||||||||||
|
|||||||||||||||||
6. Complete the following sentence: |
|||||||||||||||||
| A carbon-12 nucleus has a(n) mass compared to the total mass of the separated individual nucleons. | |||||||||||||||||
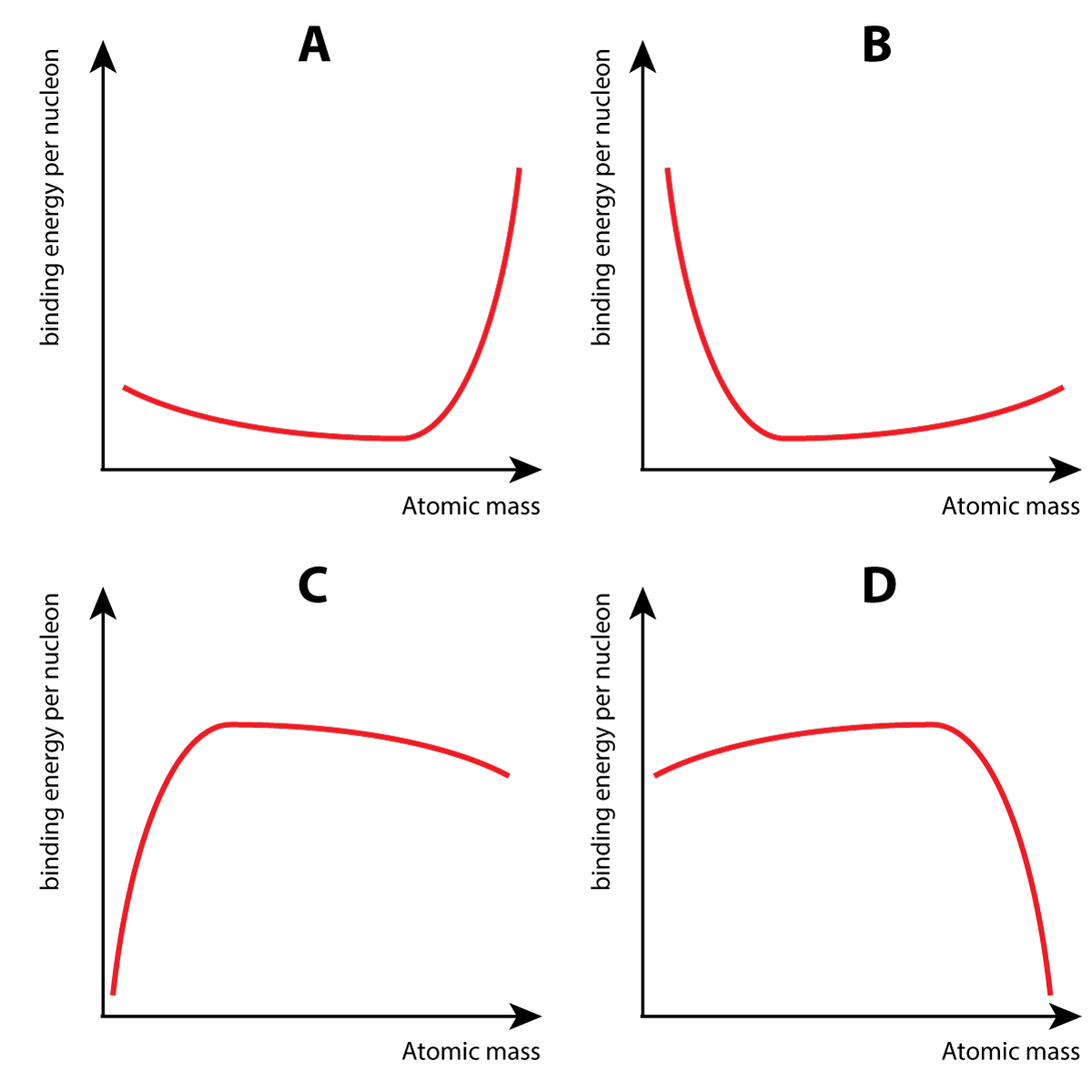
7. Binding energy per nucleon varies with atomic mass and can be compared on a graph as shown below. Which of the graphs below gives the approximate shape of the best fit line of this graph? |
|||||||||||||||||
 | |||||||||||||||||
8-10. These questions are about this element lithium. The symbol is shown here :
To the nearest whole number, the binding energy per nucleon of lithium-7 is 6 MeV. |
Pieces of Lithium metal from the Dennis s.k collection - Wikimedia |
||||||||||||||||
8. What are the values of N, Z and A for this stable isotope of lithium?
| |||||||||||||||||
9. What is the total mass defect of a lithium-7 nuclei using the value given above?
| |||||||||||||||||
10. If two lithium-7 nuclei fuse together, they would produce an isotope of carbon called carbon-14. The binding energy per nucleon for carbon-14 is 8 MeV, to the nearest whole number. Using the rounded (whole number) data given, what energy is released if two lithium-7 nuclei fuse to produce a carbon-14 nucleus? |
|
||||||||||||||||
| |||||||||||||||||