10 minutes maximum! Can you do it in 5? |
|||||||||||||||||
Q1+2. When a nucleus emits an α or β particle, it changes to a new element. 1. In this ß decay equation, what are the missing numbers x and y? |
|||||||||||||||||
|
|||||||||||||||||
2. In the above equation, a particle is missing from the right-hand side of the equation, having no mass or charge. What is the name of this particle?
|
|||||||||||||||||
|
3. The half life of an isotope is found to be approximately 1000 years. In a sample, only one eighth (12.5%) of the sample remains, the rest having decayed to a new element.
How old is the sample?
|
|||||||||||||||||
4. A weak radioactive source has a measured count rate of 240 counts per minute, and a half life of 3 hrs. However the background count in the lab is 40 counts per minute and needs to be taken into consideration. What will be the expected measured count rate per minute after 6 hours?
| |||||||||||||||||
5. An isotope X is shown on the graph below, with A=222 and Z=86. The isotope emits an alpha particle followed by a beta particle. Which letter A to D shows the final position of the isotope?
|
|||||||||||||||||
6. Radioactivity is random and spontaneous. Which of the following best describes these words in this context?
|
|||||||||||||||||
7. A patient in a hospital is injected with a radioactive isotope. Special cameras outside the body can follow the path of the isotope to diagnose medical conditions. This is known as a medical tracer. The isotope should give out a specific type of nuclear radiation, and have a suitable half life. Which of the following options is most suitable? |
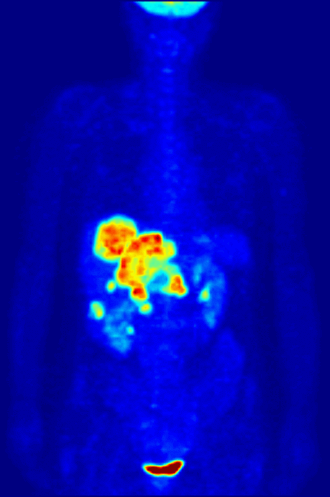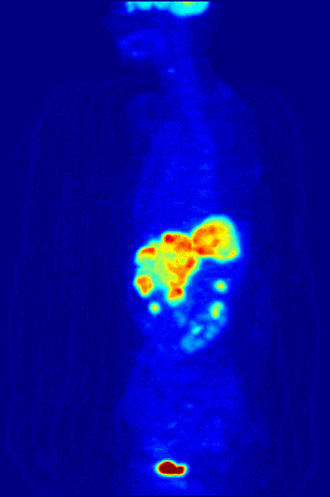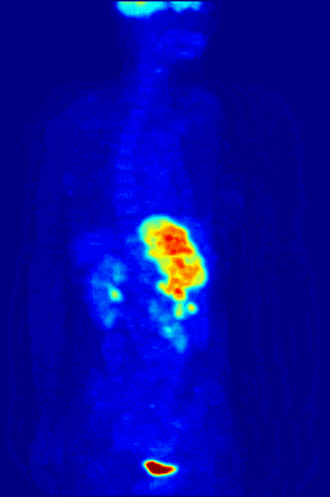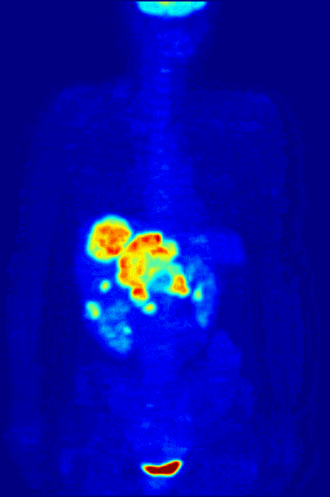 |
||||||||||||||||
| |||||||||||||||||
8+9. Aluminium foil is made using 2 rollers that squeeze the aluminium into a thin sheet. A radioactive source and a detector are used either side of the sheet to measure how thick the foil is, and make automatic adjustments to the rollers if needed. |
|||||||||||||||||
|
|||||||||||||||||
8. Which type of radiation should be emitted by the source?
| |||||||||||||||||
9. The half-life of the source needs to be suitable to stop the detector making unnecessary changes to the rollers. How long should the half-life be, and what would happen to the foil thickness if it was the wrong length half-life?
| |||||||||||||||||
10. Carbon 14 is used to date radioactive samples. In one sample, only 2 mg of carbon-14 remains, the rest having decayed into 14 mg of stable nitrogen. The half life of carbon 14 is 5700 yrs. How old is the sample?
| |||||||||||||||||